Project’s Concept
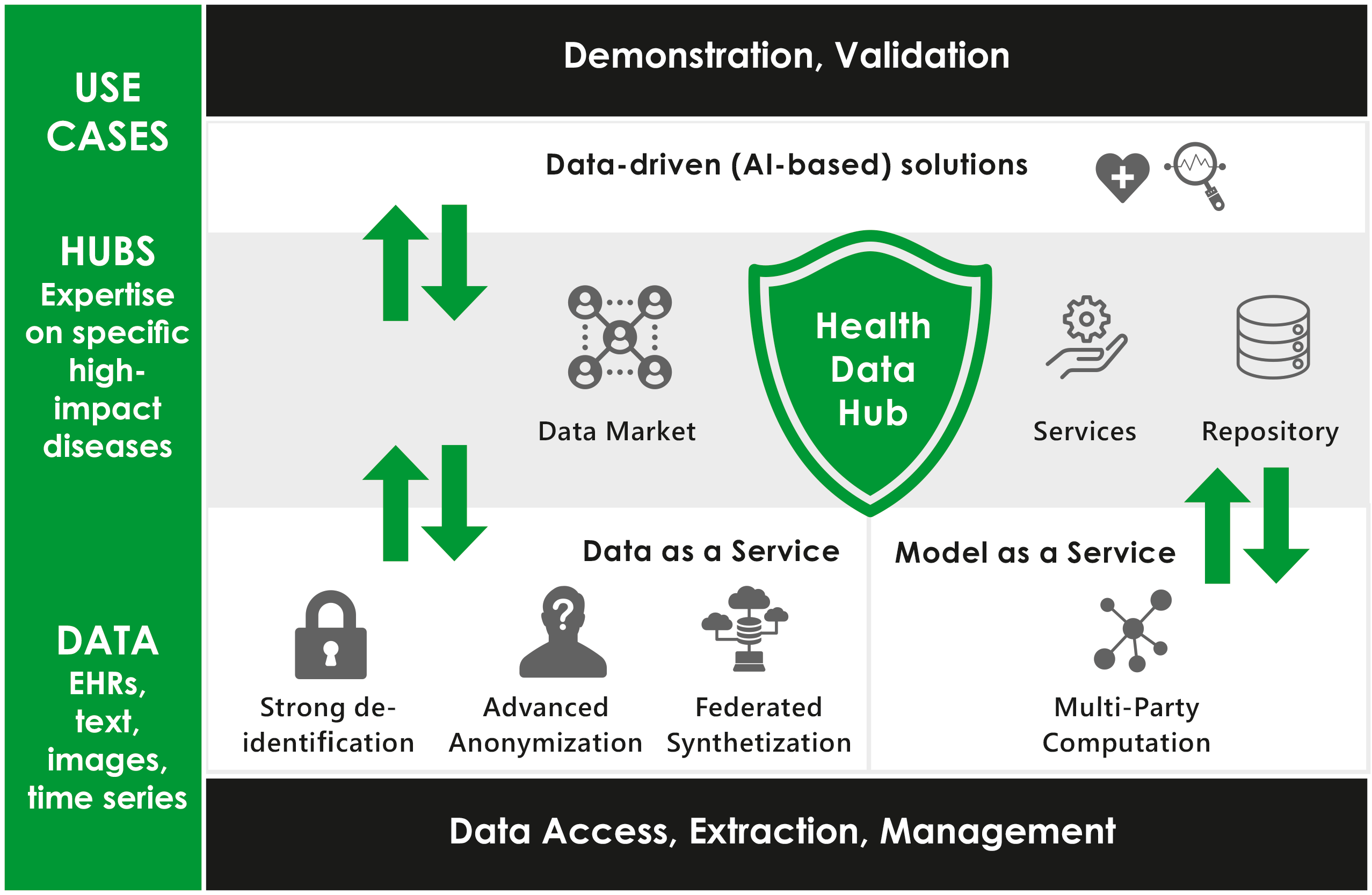
PHASE IV AI will provide and validate a comprehensive set of scientific, technological, and enabling results accessible as services. Beyond the mere aspects of research on beyond SotA technology, the accessibility and applicability of the technological results are key issues for competitiveness of the European Health industry and citizens, health care providers and health systems benefiting from a swift uptake of innovative health technologies and services. With the holistic approach of PHASE IV A ,researchers, innovators as well as the European health industry shall gain access to privacy compliant data and computation as a service in high quality facilitating decreased time-to-market for data-driven innovation increasing its competitiveness.
Therefore, PHASE IV AI follows an agile integrated approach developing (1) data synthetization services (DaaS), (2) multi-party computation services (MaaS), and (3) a Health Data Hub. Underlying to these developments, innovative tools for in-situ data extraction, data management, e.g. filtering, cleansing, and joining, shall be utilized. The development of PHASE IV AI will setup upon clinical data environments across several European regions – Scandinavia, Central Europe, Western Europe, and the United Kingdom. This way a wide variety of data can be accessed as well as a broad validation of the developed tools and services along diverse work environments and professional user groups is ensured.


